Face-centered Cubic on:
[Wikipedia]
[Google]
[Amazon]

 In
In
 As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the
As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the
 One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
 It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the
It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the
 The
The
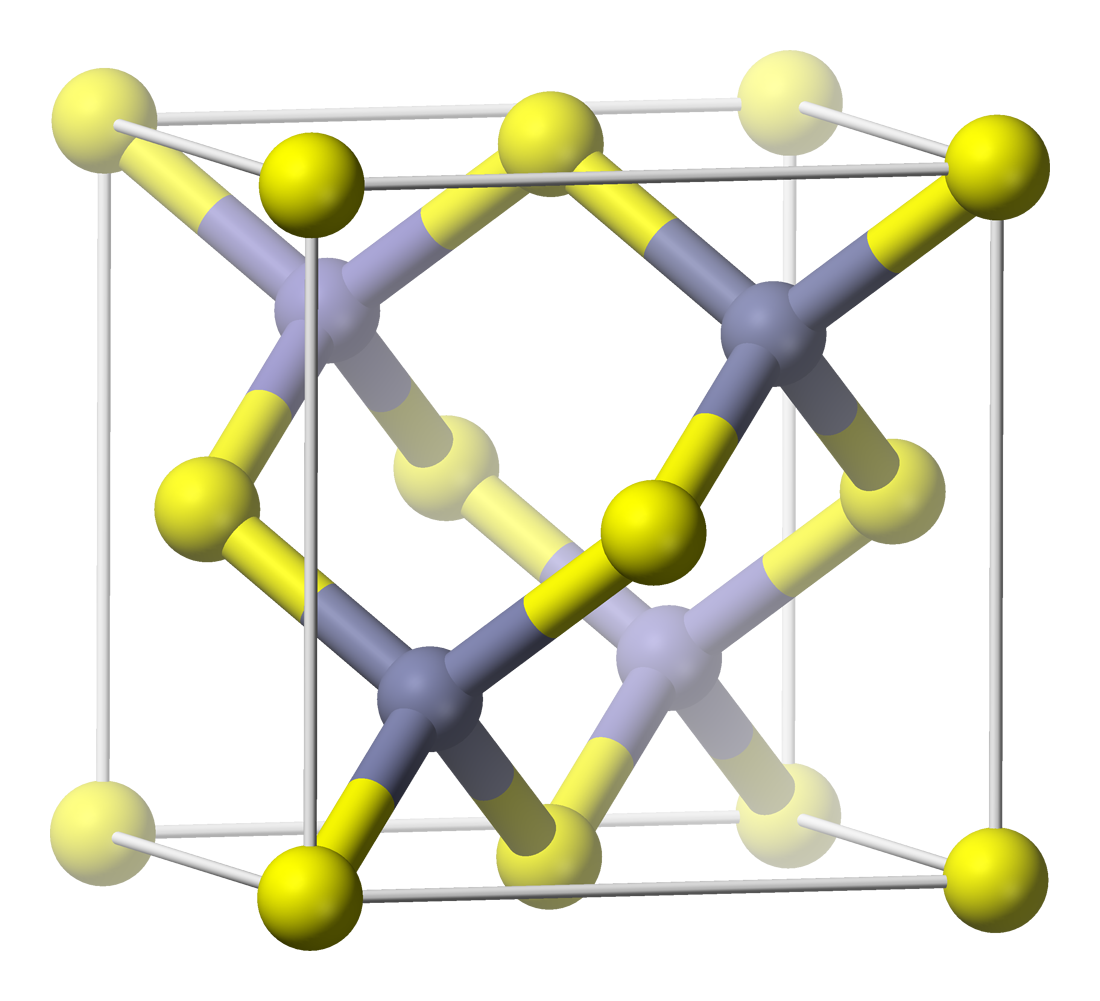 The
The 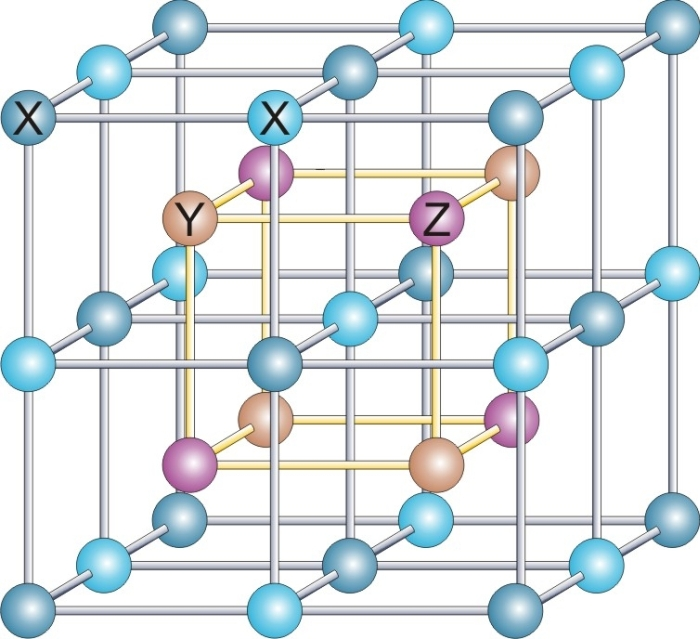
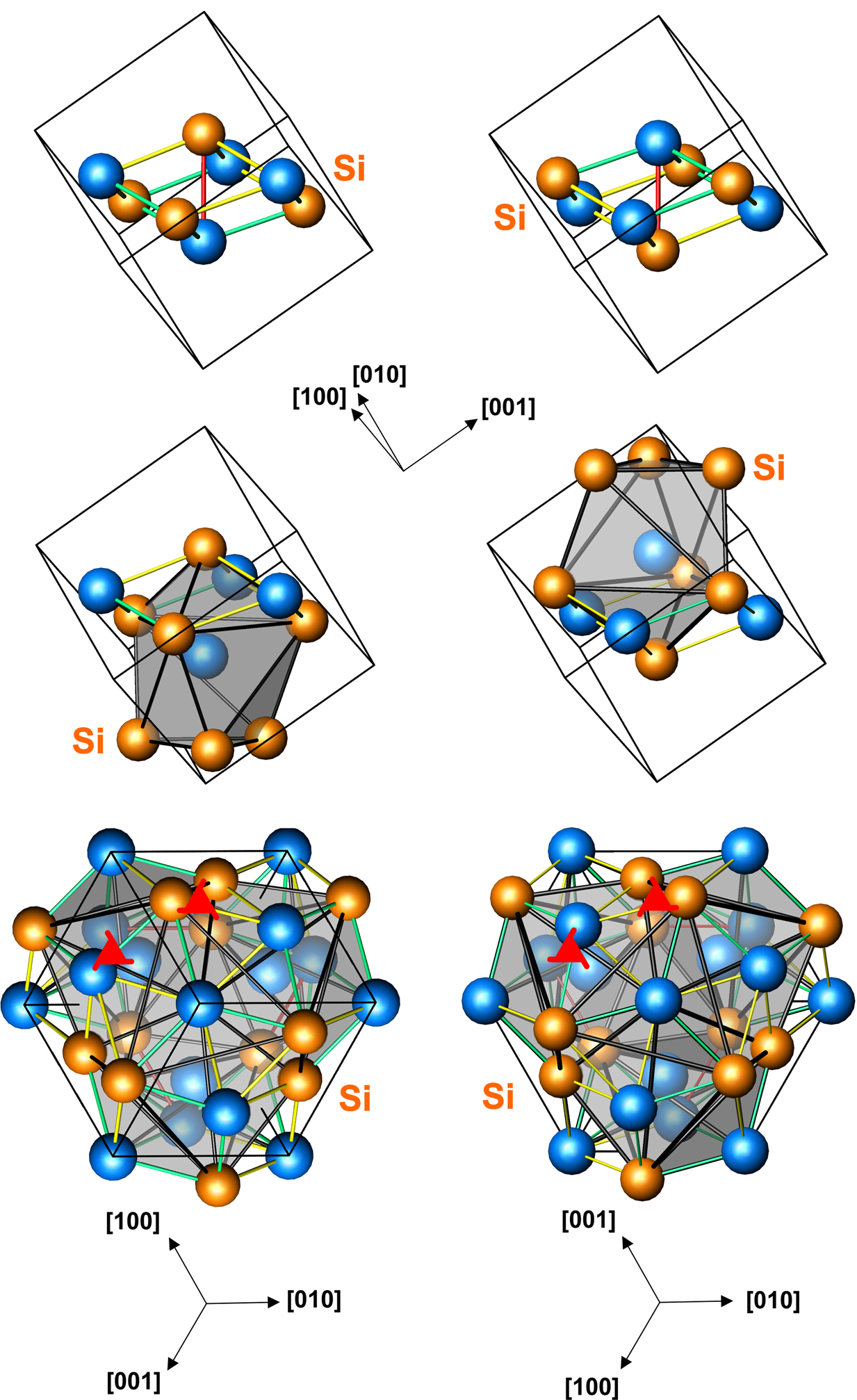 The space group of the iron monosilicide structure is P213 (No. 198), and the Strukturbericht designation is B20. This is a
The space group of the iron monosilicide structure is P213 (No. 198), and the Strukturbericht designation is B20. This is a
 A
A
Simple cubic
BCC
FCC
HCP
Making crystal structure
with Molview {{DEFAULTSORT:Cubic Crystal System Crystal systems Cubes
crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
, the cubic (or isometric) crystal system is a crystal system
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their po ...
where the unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
is in the shape of a cube
In geometry, a cube is a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex. Viewed from a corner it is a hexagon and its net is usually depicted as a cross.
The cube is the only r ...
. This is one of the most common and simplest shapes found in crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s and mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ( ...
s.
There are three main varieties of these crystals:
*Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic)
*Body-centered cubic (abbreviated ''cI'' or bcc)
*Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp)
Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not.
Bravais lattices
The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of onelattice
Lattice may refer to:
Arts and design
* Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material
* Lattice (music), an organized grid model of pitch ratios
* Lattice (pastry), an orna ...
point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes, and the unit cell therefore contains in total one atom ( × 8).
The body-centered cubic lattice (cI) has one lattice point in the center of the unit cell in addition to the eight corner points. It has a net total of two lattice points per unit cell ( × 8 + 1).
The face-centered cubic lattice (cF) has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell ( × 8 from the corners plus × 6 from the faces).
The face-centered cubic lattice is closely related to the hexagonal close packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
(hcp) system, where two systems differ only in the relative placements of their hexagonal layers. The 11plane of a face-centered cubic lattice is a hexagonal grid.
Attempting to create a base-centered cubic lattice (i.e., putting an extra lattice point in the center of each horizontal face) results in a simple tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square ...
Bravais lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
.
Coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
(CN) is the number of nearest neighbors of a central atom in the structure. Each sphere in a cP lattice has coordination number 6, in a cI lattice 8, and in a cF lattice 12.
Atomic packing factor In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity. In atomi ...
(APF) is the fraction of volume that is occupied by atoms. The cP lattice has an APF of about 0.524 , the cI lattice an APF of about 0.680, and the cF lattice an APF of about 0.740.
Crystal classes
The ''isometric crystal system'' class names, point groups (inSchönflies notation The Schoenflies (or Schönflies) notation, named after the German mathematician Arthur Moritz Schoenflies, is a notation primarily used to specify point groups in three dimensions. Because a point group alone is completely adequate to describe the ...
, Hermann–Mauguin notation
In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...
, orbifold
In the mathematical disciplines of topology and geometry, an orbifold (for "orbit-manifold") is a generalization of a manifold. Roughly speaking, an orbifold is a topological space which is locally a finite group quotient of a Euclidean space.
D ...
, and Coxeter notation
In geometry, Coxeter notation (also Coxeter symbol) is a system of classifying symmetry groups, describing the angles between fundamental reflections of a Coxeter group in a bracketed notation expressing the structure of a Coxeter-Dynkin diagram ...
), type, examples, international tables for crystallography space group number, and space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
s are listed in the table below. There are a total 36 cubic space groups.
Other terms for hexoctahedral are: normal class, holohedral, ditesseral central class, galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
type.
Single element structures
orbital hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
demands certain bond angles
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determ ...
.) Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character ...
. The ''bcc'' and ''fcc'', with their higher densities, are both quite common in nature. Examples of ''bcc'' include iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
, chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
, and niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
. Examples of ''fcc'' include aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
.
Another important cubic crystal structure is the diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the sem ...
structure, which can appear in carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
, germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, and tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
. Unlike fcc and bcc, this structure is not a lattice, since it contains multiple atoms in its primitive cell. Other cubic elemental structures include the A15 structure found in tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
, and the extremely complicated structure of manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
.
Multi-element structures
Compounds that consist of more than one element (e.g.binary compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended soli ...
s) often have crystal structures based on the cubic crystal system. Some of the more common ones are listed here. These structures can be viewed as two or more interpenetrating sublattices where each sublattice occupies the interstitial site
In crystallography, interstitial sites, holes or voids are the empty space that exists between the packing of atoms (spheres) in the crystal structure.
The holes are easy to see if you try to pack circles together; no matter how close you get ...
s of the others.
Caesium chloride structure
 One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
 It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the
It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
when prepared at low temperatures or high pressures.Seitz, ''Modern Theory of Solids'' (1940), p.49 Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
of the caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
(CsCl) structure is called Pmm (in Hermann–Mauguin notation
In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...
), or "221" (in the International Tables for Crystallography). The Strukturbericht designation is "B2".
There are nearly a hundred rare earth intermetallic compounds
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
that crystalize in the CsCl structure, including many binary compounds
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended soli ...
of rare earths with magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, and with elements in groups 11, 12, and 13. Other compounds showing caesium chloride like structure are CsBr, CsI, high-temperature RbCl
Ribulose-1,5-bisphosphate carboxylase-oxygenase, commonly known by the abbreviations RuBisCo, rubisco, RuBPCase, or RuBPco, is an enzyme () involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is con ...
, AlCo, AgZn, BeCu, MgCe, RuAl and SrTl.
Rock-salt structure
space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
of the rock-salt or halite
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, p ...
(sodium chloride) structure is denoted as Fmm (in Hermann–Mauguin notation
In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...
), or "225" (in the International Tables for Crystallography). The Strukturbericht designation is "B1".
In the rock-salt structure, each of the two atom types forms a separate face-centered cubic lattice, with the two lattices interpenetrating so as to form a 3D checkerboard pattern. The rock-salt structure has octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
coordination: Each atom's nearest neighbors consist of six atoms of the opposite type, positioned like the six vertices of a regular octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
. In sodium chloride there is a 1:1 ratio of sodium to chlorine atoms. The structure can also be described as an FCC lattice of sodium with chlorine occupying each octahedral void or vice versa.
Examples of compounds with this structure include sodium chloride itself, along with almost all other alkali halides, and "many divalent metal oxides, sulfides, selenides, and tellurides". According to the radius ratio rule, this structure is more likely to be formed if the cation is somewhat smaller than the anion (a cation/anion radius ratio of 0.414 to 0.732).
The interatomic distance (distance between cation and anion, or half the unit cell length ''a'') in some rock-salt-structure crystals are: 2.3 Å (2.3 × 10−10 m) for NaF, 2.8 Å for NaCl, and 3.2 Å for SnTe. Most of the alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s and halides have the rock salt structure, though a few have the caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
structure instead.
Many transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
monoxides also have the rock salt structure ( TiO, VO, CrO, MnO, FeO
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists ...
, CoO
COO or coo may refer to:
Business
* Certificate of origin, used in international trade
* Chief operating officer or chief operations officer, high-ranking corporate official
* Concept of operations, used in Systems Engineering Management Process
...
, NiO
are two wrathful and muscular guardians of the Buddha standing today at the entrance of many Buddhist temples in East Asian Buddhism in the form of frightening wrestler-like statues. They are dharmapala manifestations of the bodhisattva Vajra ...
, CdO). The early actinoid monocarbides also have this structure (ThC
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC' ...
, PaC, UC, NpC, PuC). Other compounds showing rock salt like structure are TiB, ZrB, PbS
The Public Broadcasting Service (PBS) is an American public broadcasting, public broadcaster and Non-commercial activity, non-commercial, Terrestrial television, free-to-air television network based in Arlington, Virginia. PBS is a publicly fu ...
, PbSe, PbTe, SnTe, AgF, AgCl, and AgBr.
Fluorite structure
Much like the rock salt structure, thefluorite structure In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure ...
(AB2) is also an Fmm structure but has 1:2 ratio of ions. The anti-fluorite structure is nearly identical, except the positions of the anions and cations are switched in the structure. They are designated Wyckoff positions
In crystallography, a Wyckoff position is a point belonging to a set of points for which site symmetry groups are conjugate subgroups of the space group. Crystallography tables give the Wyckoff positions for different space groups.
For any point i ...
4a and 8c whereas the rock-salt structure positions are 4a and 4b.
Zincblende structure
 The
The space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
of the Zincblende structure is called F3m (in Hermann–Mauguin notation
In geometry, Hermann–Mauguin notation is used to represent the symmetry elements in point groups, plane groups and space groups. It is named after the German crystallographer Carl Hermann (who introduced it in 1928) and the French mineralogis ...
), or 216. The Strukturbericht designation is "B3".
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimen ...
), one form of zinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various ...
(β-ZnS). As in the rock-salt structure, the two atom types form two interpenetrating face-centered cubic lattices. However, it differs from rock-salt structure in how the two lattices are positioned relative to one another. The zincblende structure has tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
coordination: Each atom's nearest neighbors consist of four atoms of the opposite type, positioned like the four vertices of a regular tetrahedron
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
. In zinc sulfide the ratio of zinc to sulfur is 1:1. Altogether, the arrangement of atoms in zincblende structure is the same as diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the sem ...
structure, but with alternating types of atoms at the different lattice sites. The structure can also be described as an FCC lattice of zinc with sulfur atoms occupying half of the tetrahedral voids or vice versa.
Examples of compounds with this structure include zincblende itself, lead(II) nitrate
Lead(II) nitrate is an inorganic compound with the chemical formula Pb( NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumbu ...
, many compound semiconductors (such as gallium arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure.
Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monoli ...
and cadmium telluride
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with ca ...
), and a wide array of other binary compounds. The boron group
The boron group are the chemical elements in group 13 of the periodic table, comprising boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and nihonium (Nh). The elements in the boron group are characterized by having three vale ...
pnictogenide
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the el ...
s usually have a zincblende structure, though the nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant ...
s are more common in the wurtzite structure
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crysta ...
, and their zincblende forms are less well known polymorphs.
This group is also known as the II-VI family of compounds, most of which can be made in both the zincblende (cubic) or wurtzite
Wurtzite is a zinc and iron sulfide mineral with the chemical formula , a less frequently encountered Polymorphism (materials science), structural polymorph form of sphalerite. The iron content is variable up to eight percent.Palache, Charles, Har ...
(hexagonal) form.
This group is also known as the III-V family of compounds.

Heusler structure
The Heusler structure, based on the structure of Cu2MnAl, is a common structure forternary compound
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements.
While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases r ...
s involving transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
. It has the space group Fmm (No. 225), and the Strukturbericht designation is L21. Together with the closely related half-Heusler and inverse-Huesler compounds, there are hundreds of examples.
Iron monosilicide structure
 The space group of the iron monosilicide structure is P213 (No. 198), and the Strukturbericht designation is B20. This is a
The space group of the iron monosilicide structure is P213 (No. 198), and the Strukturbericht designation is B20. This is a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
structure, and is sometimes associated with helimagnetic
Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition be ...
properties. There are four atoms of each element for a total of eight atoms in the unit cell.
Examples occur among the transition metal silicides and germanides, as well as a few other compounds such as gallium palladide
Gallium palladide (GaPd or PdGa) is an intermetallic combination of gallium and palladium. In the Iron monosilicide crystal structure. The compound has been suggested as an improved Hydrogenation#Catalysts, catalyst for hydrogenation reactions. In ...
.
Weaire–Phelan structure
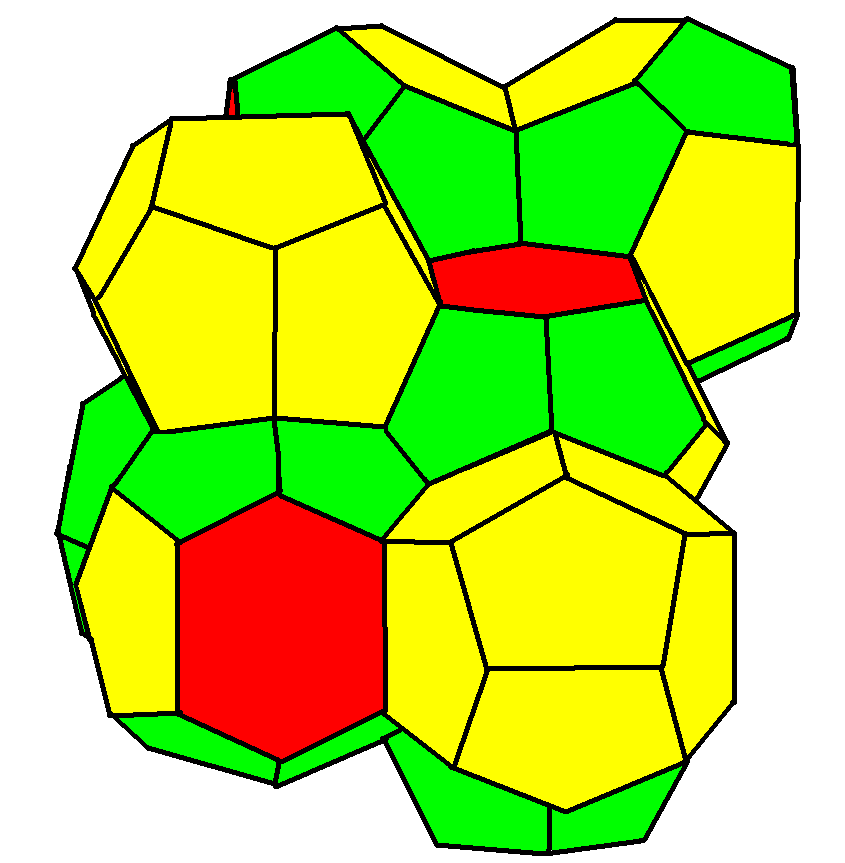
 A
A Weaire–Phelan structure
In geometry, the Weaire–Phelan structure is a three-dimensional structure representing an idealised foam of equal-sized bubbles, with two different shapes. In 1993, Denis Weaire and Robert Phelan found that this structure was a better solution ...
has Pmn (223) symmetry.
It has three orientations of stacked tetradecahedron
240px, A tetradecahedron with ''D2d'' symmetry, existing in the Weaire–Phelan structure
A tetradecahedron is a polyhedron with 14 faces. There are numerous topologically distinct forms of a tetradecahedron, with many constructible entirely wi ...
s with pyritohedral
image:tetrahedron.jpg, 150px, A regular tetrahedron, an example of a solid with full tetrahedral symmetry
A regular tetrahedron has 12 rotational (or orientation-preserving) symmetries, and a symmetry order of 24 including transformations that c ...
cells in the gaps. It is found as a crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
in chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
where it is usually known as a "type I clathrate
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin (), meaning ‘with bars, latticed’. Most clathrate compounds are polymeric and completely envelop t ...
structure". Gas hydrates
Clathrate hydrates, or gas hydrates, clathrates, hydrates, etc., are crystalline water-based solids physically resembling ice, in which small non-polar molecules (typically gases) or polar molecules with large hydrophobic moieties are trapped i ...
formed by methane, propane, and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ed together, and the larger gas molecules are trapped in the polyhedral cages.
See also
*Atomium
The Atomium ( , , ) is a landmark building in Brussels, Belgium, originally constructed for the 1958 Brussels World's Fair (Expo '58). It is located on the Heysel/Heizel Plateau in Laeken (northern part of the City of Brussels), where the exh ...
: building which is a model of a ''bcc'' unit cell, with vertical body diagonal.
*Close-packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
*Dislocations
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sl ...
*Reciprocal lattice
In physics, the reciprocal lattice represents the Fourier transform of another lattice (group) (usually a Bravais lattice). In normal usage, the initial lattice (whose transform is represented by the reciprocal lattice) is a periodic spatial fu ...
References
Further reading
*Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., Wiley,External links
*JMol
Jmol is computer software for molecular modelling chemical structures in 3-dimensions. Jmol returns a 3D representation of a molecule that may be used as a teaching tool, or for research e.g., in chemistry and biochemistry.
It is written in the ...
simulations by Graz University:
Simple cubic
BCC
FCC
HCP
Making crystal structure
with Molview {{DEFAULTSORT:Cubic Crystal System Crystal systems Cubes